Human pluripotent stem cells (hPSCs) come with unique characteristics that require careful consideration when developing an effective Quality Control workflow. Last month, drawing on years of experience supporting hundreds of hPSC scientists worldwide, Stem Genomics hosted a webinar highlighting the key challenges we’ve encountered in hPSC quality control. Here’s a preview of what we covered:
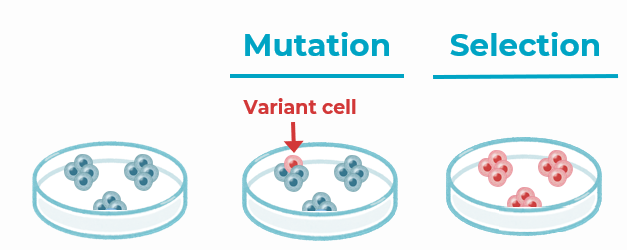
1. hPSCs can acquire genetic abnormalities during culture every 5 to 10 passages
- Most of these abnormalities are CNVs (Copy Number Variants).
- They are non-random.
- Stressful processes such as reprogramming or gene editing favour their appearance in cultures.
- The abnormalities provide the cells with specific selective advantages such as increased proliferation, inhibited apoptosis or reduced differentiation capacity.
(Dubose et al., 2022; Hastings et al., 2009)
2. The most frequent genomic abnormality found in hPSC cultures is the 20q11.21
- It represents >20% of defects found in hPSCs.
- It involves the BCL2L1 gene.
- With a size of 0.9 Mb, it is a sub-karyotyping abnormality that falls under the scope of detection of G-Banding.
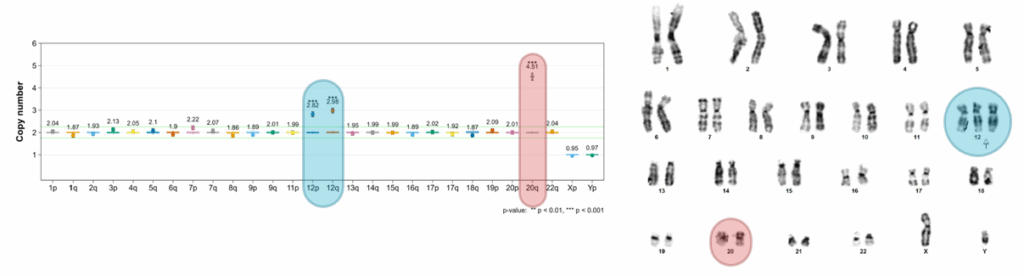
On the left: digital PCR-based iCS-digitalTM PSC results revealing a significant abnormality on chromosome 20q. On the right: the same sample analyzed by G-banding, which fails to detect the 20q abnormality due to limited resolution (source: Stem Genomics platform).
3. hPSC lines, just like other cell lines, are susceptible to misidentification
- The proportion of misidentified cell lines worldwide is 1/5 to 1/3 of the registered cell lines (source: ICLAC).
- This originates from cross-contamination and mislabeling. It leads to inconsistent nomenclature.
- HeLa (cancer cell-line) is the most common cross contaminant found in around 26% of the misidentified lines.
- The impact of misidentification is massive: $28 billion dollars per year have been attributed to the use of unauthenticated cell lines. (Freedman et al., 2015)
4. Mycoplasma detection remains critical for hPSC cultures
- An alarming high average of 35% of contamination in laboratories worldwide (as high as 60% in Japan!).
- Mycoplasma is not visible through the microscope and not sensitive to antibiotics.
(Corral-Vázquez et al., 2017; Drexler et al., 2017)
The challenge for hPSC scientists is developing an affordable and efficient QC workflow that addresses all these considerations while meeting international regulations and applicable standards. Our webinar offers a practical solution based on Stem Genomics’ expertise.


