> Range of assays > Identity: Cell line authentication
Identity: Cell line authentication
Ensure consistent cell line identity throughout your culture
The risks of cross-contamination in the laboratory are high. Mislabeling can happen too. In fact, The International Cell Line Authentication Committee (ICLAC) estimates that as much as 1/5 to 1/3 of known cell lines are wrongly identified to date (2021 data).
There is no obvious visual evidence that will flag this issue up to you without testing for identity. Most of the time, scientists are misled because their cell line behaves the way they would expect. Still, this does not mean that the cell line identity has not changed during the culture process.
This will cause serious issues down the line, compromising the robustness of published results and the credibility of the field overall. It is also ethically fundamental to ensure the consented agreement from the initial donor of the line on which you are basing your research work.
Fortunately, awareness of this issue is rising in the field of stem cell research. An increasing number of journals are now asking for the test to be performed before publishing papers. It also helps in preparing the ground for future clinical trials that will require stringent quality controls from the proof of concept to the actual production material. Finally, a proper cell line authentication using STR will also help you meet the requirements for grants and funding applications.
Discover our cell identification service in video
Watch a short presentation video explaining all
there is to know about the cell authentication service
by Stem Genomics.
Find an easy, fast and affordable STR solution with Stem Genomics
The latest ISSCR guidelines published in June 2023 (Standards for Human Stem Cell Use in Research), encourage the use of STR tests at regular intervals on your production workflow.
The ISSCR encourages checking your cell line identity at the initial characterization of the Master Cell Bank, at significant bottleneck (for eg. after gene editing) and at the end of the study.
In addition, Stem Genomics recommends testing at the acquisition of a new cell line (unless an authentication certificate is provided). Regular in-routine controls during amplification are also good practice to minimize risks of cross-contamination and mislabelling.
Click on the graphs below for more details
Guidelines for human Pluripotent Stem cell workflow
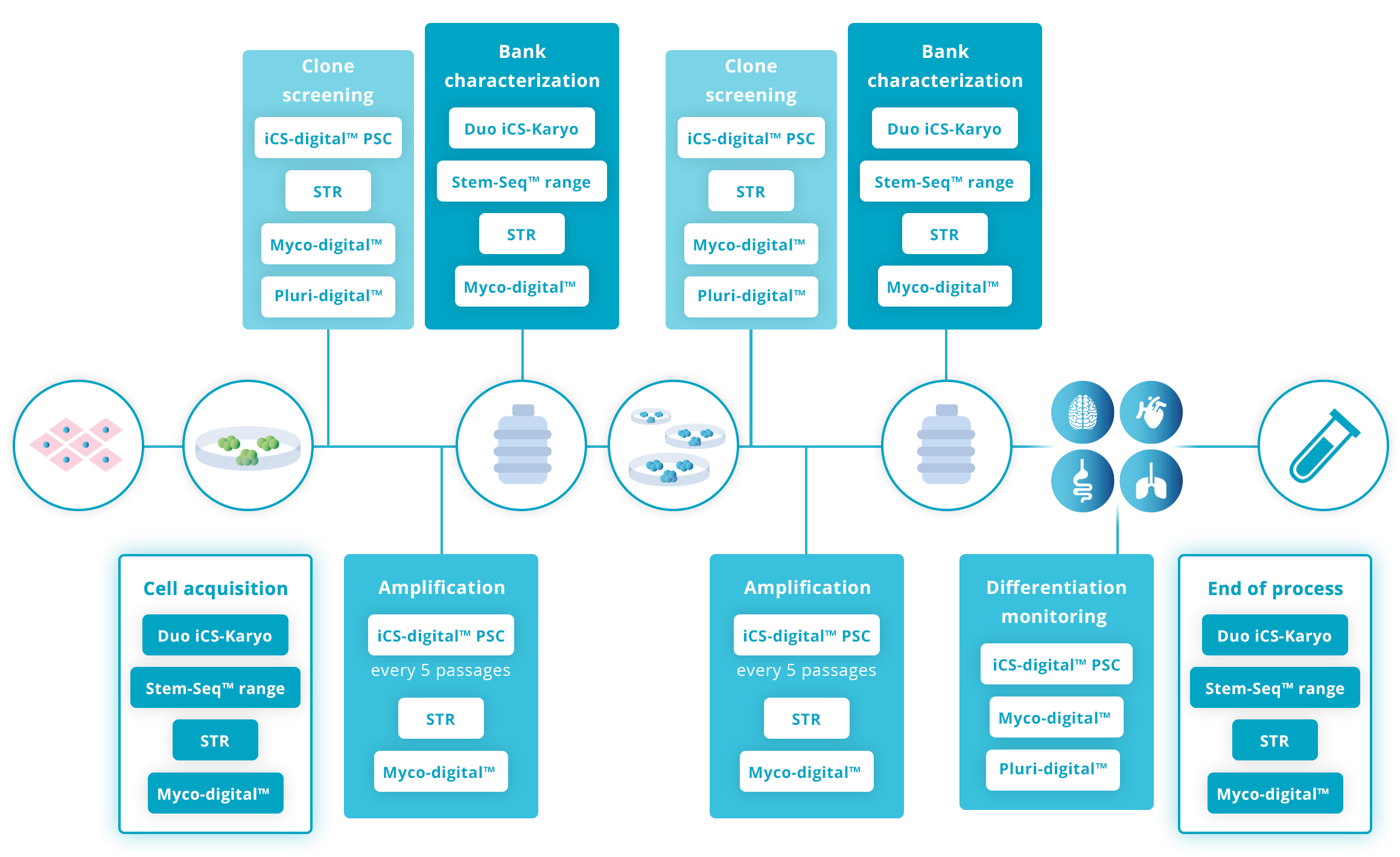
Guidelines for human Mesenchymal Stromal cell workflow
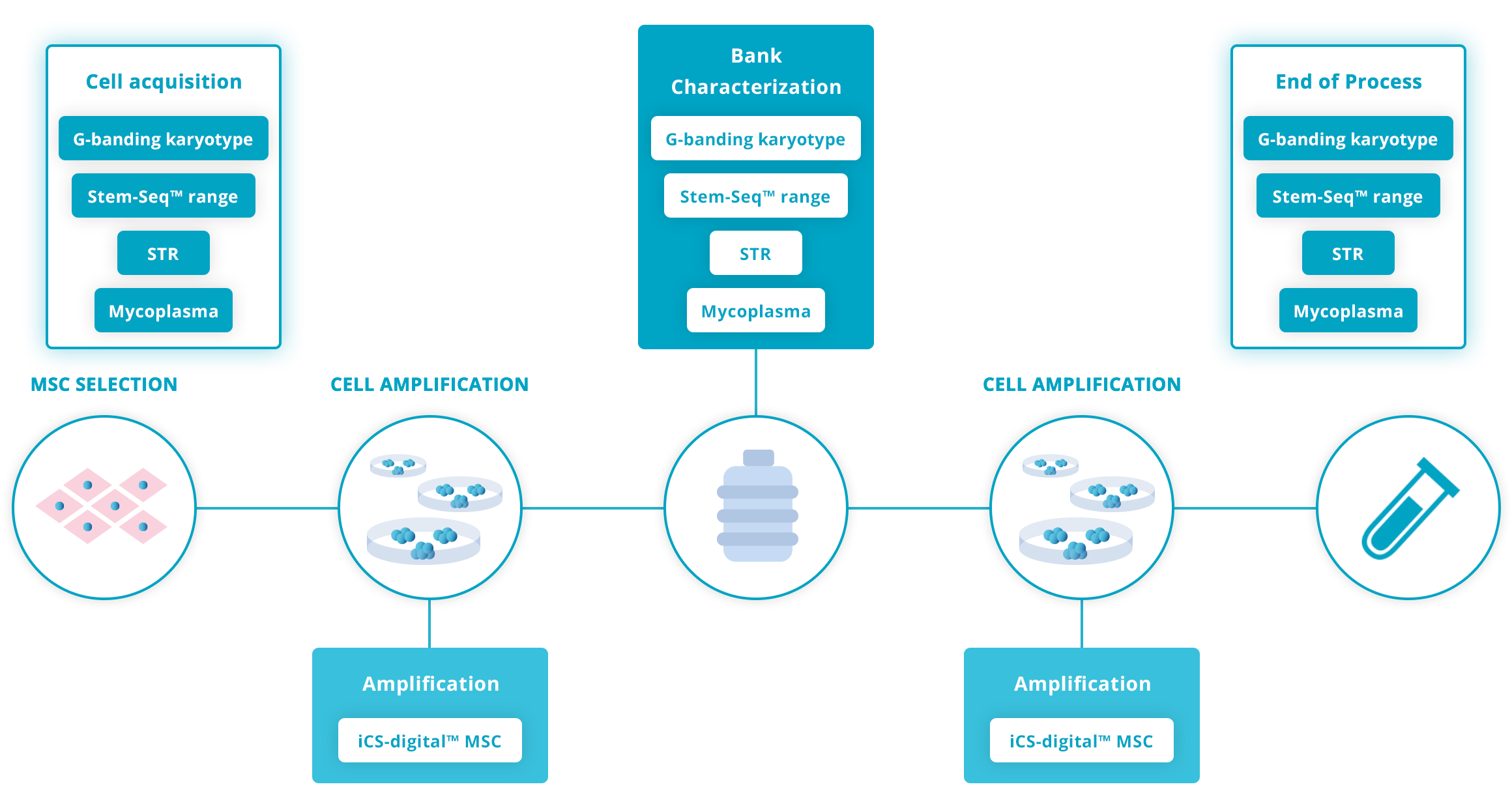
Amplification
Amplification
STR-solution by Stem Genomics: your results in 3 days
Get your cell lines properly authenticated with the STR-solution from Stem Genomics. Simply send your sample* in the post and you will get fast results within 3 business days**.
*analysis available from 1 sample
** 3 business days for Europe, 7 business days for the USA and Canada.
STR by Stem Genomics specifications at a glance
Any human cell type
gDNA
Cell pellet
Room temperature
Dry ice
5-Dye
16-Locus analysis
3 days after sample reception for Europe, 7 days for the USA and Canada.
Available as a service only
from one sample:
Save time and money with our one-stop shop solution
While we are verifying your cell line identity, why not also check for potential genomic abnormalities at the same time? We have fast and high-performing digital PCR tests that will enable you to perform these tests simultaneously from the same sample.
Watch a quick video presentation of the one-stop-shop service.
Need help?
Our experts specialized in stem cell characterization are here to help and recommend the procedure best adapted to your specific workflow.

