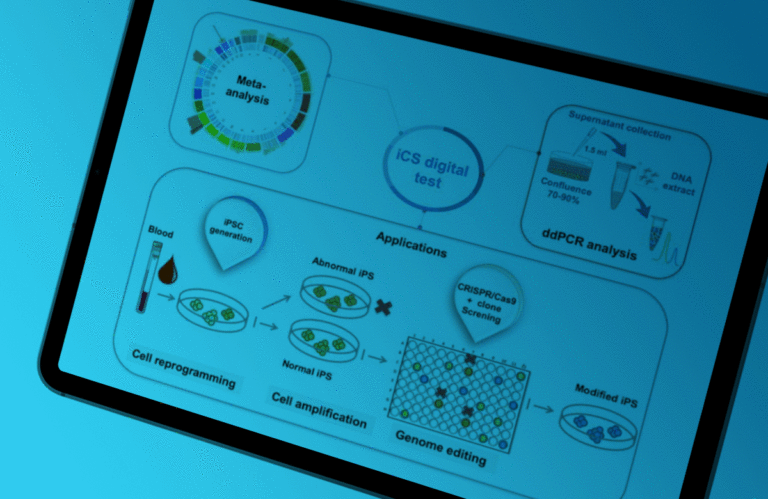
Recurrent Genetic Abnormalities in Human Pluripotent Stem Cells: Definition and Routine Detection in Culture Supernatant by Targeted Droplet Digital PCR
In the article published in Stem Cell Reports in January 2020, Assou et al. demonstrate the interest of the iCS-digital™ test for routine human pluripotent stem cell (hPSC) screening. Thanks to the specificity of the digital PCR (dPCR) probe panel, the simplicity of sample collection, and the accuracy of the ddPCR technology, this test allows the fast and straightforward monitoring of hPSC integrity.
This innovative test can change how hPSC quality controls are implemented for basic research and cell therapy by increasing the test frequency to secure cell productions and avoid expensive and time-consuming cell cultures.
The iCS-digital™ test is exclusively provided by Stem Genomics as a full service.